- For US Healthcare Professionals Only
- Important Safety
Information - Indication
- Prescribing
Information - Patient
Website
DISTRIBUTION
NETWORK
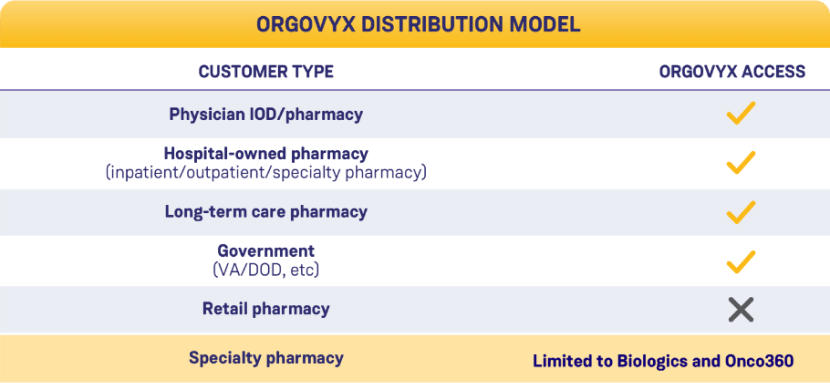
What pharmacies are eligible
to order ORGOVYX?

Join over 5000 healthcare professionals who have prescribed ORGOVYX since 20211*
*Based on claims and specialty pharmacy data from healthcare professionals across specialties from June 2021 to May 2023.1
IMPORTANT INFORMATION FOR ELIGIBLE PHARMACIES
If a patient’s insurance benefit requires them to use an ineligible specialty pharmacy, contact the pharmacy benefits manager (PBM) to request an override.
If your pharmacy is unable to dispense a prescription because it does not have a pharmacy network contract, you can transfer the prescription to 1 of the 2 ORGOVYX specialty pharmacies listed on this page.
ORGOVYX authorized distributors
ORGOVYX is supplied in a bottle containing 30 tablets that are 120 mg each (NDC: 72974-120-01).
ORGOVYX is available to in-office dispensing pharmacies and hospital/academic/institutional specialty pharmacies. ORGOVYX can only be ordered through the authorized specialty distributors below:
Email: service@asdhealthcare.com
Phone: 1-800-746-6273
Fax: 1-800-547-9413
Standard Ordering Hours: Monday-Thursday,
8 AM-7:30 PM ET; Friday, 8 AM-7 PM ET
Email: service@besse.com
Phone: 1-800-543-2111
Fax: 1-800-543-8695
Standard Ordering Hours: Monday-Thursday,
8:30 AM-7:30 PM ET; Friday, 8:30 AM-5 PM ET
specialtyonline.cardinalhealth.com(Physician Clinics)
orderexpress.cardinalhealth.com(Hospitals)
Email: gmb-spd-csorderentry@cardinalhealth.com
Phone: 1-855-855-0708
Fax: 1-877-274-9897
Standard Ordering Hours: Monday-Friday,
9 AM–7 PM ET
Email: customer.service@curascript.com
Phone: 1-877-599-7748
Fax: 1-800-862-6208
Standard Ordering Hours: Monday-Friday,
8 AM–7 PM ET
Email: MPBOrders@McKesson.com
Phone: 1-877-625-2566
Standard Ordering Hours: Monday-Friday,
9 AM-7:30 PM ET
Email: MSH.CustomerCare-MSPL@McKesson.com
Phone: 1-800-482-6700(Oncology)
Phone: 1-855-477-9800(Multi-specialty)
Standard Ordering Hours: Monday-Friday,
8 AM-8 PM ET
Email: service@oncologysupply.com
Phone: 1-800-633-7555
Fax: 1-800-248-8205
Standard Ordering Hours: Monday-Friday,
9 AM-8 PM ET
ORGOVYX specialty pharmacies
We have selected 2 URAC- and ACHC-accredited mail-order specialty pharmacies to provide service nationally to your patients prescribed ORGOVYX. These specialty pharmacies can also provide information and resources to your patients, including free delivery, 24-hour access to a pharmacist, ORGOVYX medication counseling, and adherence support.
Phone: 1-800-850-4306
Fax: 1-800-823-4506
Standard Ordering Hours: Monday-Friday,
8 AM-8 PM ET
Clinicians are available to speak with patients 24 hours a day, every day. See link to referral form below.
Phone: 1-877-662-6633
Fax: 1-877-662-6355
Standard Ordering Hours: Monday-Friday,
8 AM-8 PM ET
Clinicians are available to speak with patients 24 hours a day, every day. See link to referral form below.
ACHC=Accreditation Commission for Health Care; DOD=Department of Defense; IOD=in-office dispensing; URAC=Utilization Review Accreditation Commission; VA=Veterans Affairs.
Sumitomo Pharma America and Pfizer Inc. are neither affiliated with, nor do they recommend the use of, any of the listed pharmacies or distributors. It is in the prescriber’s discretion to select the appropriate pharmacy or distributor for their specific needs.
IN YOUR AREA
†This coverage information is provided for informational purposes only; individual plans vary, and this may not include all plans. Sumitomo Pharma America and Pfizer make no representation or guarantee concerning coverage or reimbursement for ORGOVYX; please check with individual payers for plan-specific coverage and reimbursement information and requirements. Nothing herein may be construed as an endorsement, approval, recommendation, representation, or warranty of any kind by any plan or insurer referenced. This information is subject to change without notice. Data on file. Formulary data are provided by MMIT, LLC, as of December 2024. Transaction data are provided by SHS database as of December 2024.1

ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or to any of the product components.
QT/QTc Interval Prolongation: Androgen deprivation therapy, such as ORGOVYX may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, or frequent electrolyte abnormalities and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Hypersensitivity: Angioedema was reported in 0.2% of patients treated with ORGOVYX in HERO. Hypersensitivity reactions, including pharyngeal edema and other serious cases of angioedema, have been reported in post-marketing with ORGOVYX. Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue ORGOVYX and promptly seek medical care. Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated.
Embryo-Fetal Toxicity: The safety and efficacy of ORGOVYX have not been established in females. Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX.
Laboratory Testing: Therapy with ORGOVYX results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after ORGOVYX may be affected. The therapeutic effect of ORGOVYX should be monitored by measuring serum concentrations of prostate-specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
Serious adverse reactions occurred in 12% of patients receiving ORGOVYX. Serious adverse reactions in ≥0.5% of patients included myocardial infarction (0.8%), acute kidney injury (0.6%), arrhythmia (0.6%), hemorrhage (0.6%), and urinary tract infection (0.5%). Fatal adverse reactions occurred in 0.8% of patients receiving ORGOVYX including metastatic lung cancer (0.3%), myocardial infarction (0.3%), and acute kidney injury (0.2%). Fatal and non-fatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX.
Most common adverse reactions (≥10%) and laboratory abnormalities (≥15%) in patients receiving ORGOVYX were hot flush (54%), glucose increased (44%), triglycerides increased (35%), musculoskeletal pain (30%), hemoglobin decreased (28%), alanine aminotransferase increased (27%), fatigue (26%), aspartate aminotransferase increased (18%), constipation (12%), and diarrhea (12%).
Co-administration of ORGOVYX with a P-gp inhibitor increases the area under the curve (AUC) and maximum concentration (Cmax) of ORGOVYX, which may increase the risk of adverse reactions associated with ORGOVYX. Avoid co-administration of ORGOVYX with oral P-gp inhibitors. If co-administration is unavoidable, take ORGOVYX first, separate dosing by at least 6 hours, and monitor patients more frequently for adverse reactions. Treatment with ORGOVYX may be interrupted for up to 2 weeks for a short course of treatment with certain P-gp inhibitors. If treatment with ORGOVYX is interrupted for more than 7 days, resume administration of ORGOVYX with a 360 mg loading dose on the first day, followed by 120 mg once daily.
Co-administration of ORGOVYX with a combined P-gp and strong CYP3A inducer decreases the AUC and Cmax of ORGOVYX, which may reduce the effects of ORGOVYX. Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers. If co-administration is unavoidable, increase the ORGOVYX dose to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX dose of 120 mg once daily.
ORGOVYX® (relugolix) is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer.
Please see full Prescribing Information for ORGOVYX.
- Data on file. Sumitomo Pharma America, Inc.
