- For US Healthcare Professionals Only
- Important Safety
Information - Indication
- Prescribing
Information - Patient
Website
HERO STUDY ADVERSE EVENTS
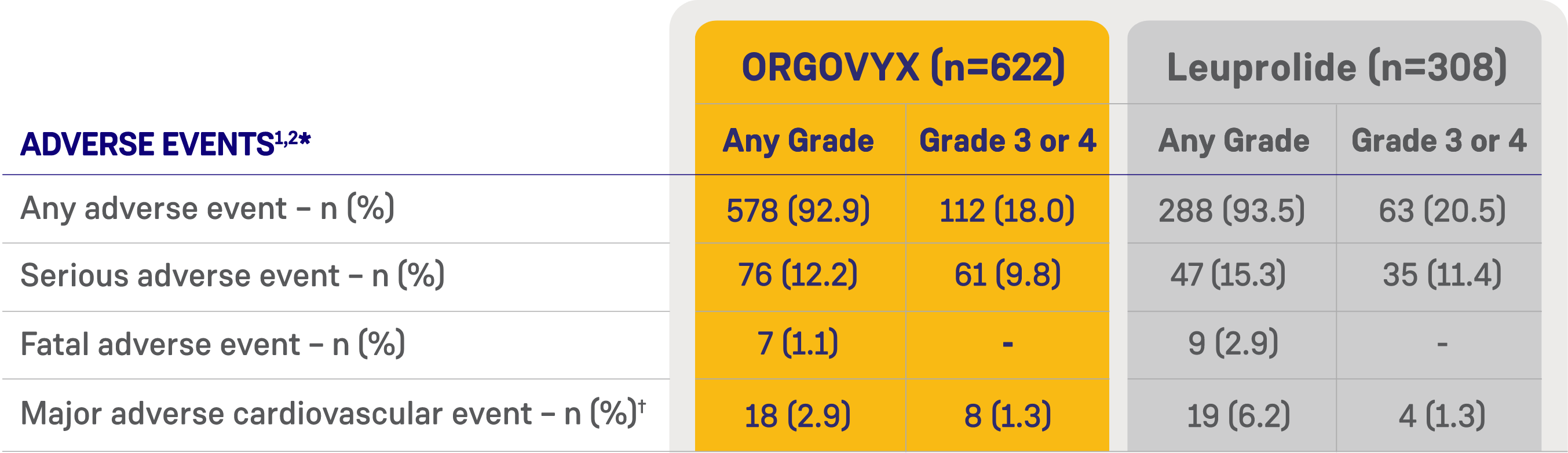
*Shown are the numbers of patients with an event, rather than the number of events. Adverse events were evaluated with the use of MedDRA, version 22.0, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.2
†Search criteria included “myocardial infarction” (broad standardized MedDRA query), “central nervous system hemorrhages and cerebrovascular conditions” (broad standardized MedDRA query), and deaths from any cause.2
MedDRA=Medical Dictionary for Regulatory Activities.
- 99 patients (16%) received concomitant radiotherapy and 17 patients (3%) received concomitant enzalutamide with ORGOVYX1
- In a prespecified safety analysis, major adverse cardiovascular events were defined as nonfatal myocardial infarction, nonfatal stroke, and all-cause death2
- In a separate analysis reported in the US Prescribing Information, fatal and nonfatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX. Fatal adverse events, excluding prostate cancer–related deaths, were reported in 0.8% of patients receiving ORGOVYX1‡
‡Reported in 2.3% of patients treated with leuprolide.3
- In a prespecified safety analysis, major adverse cardiovascular events were defined as nonfatal
myocardial infarction, nonfatal stroke, and all-cause death2
Cumulative incidence of major adverse
cardiovascular events2
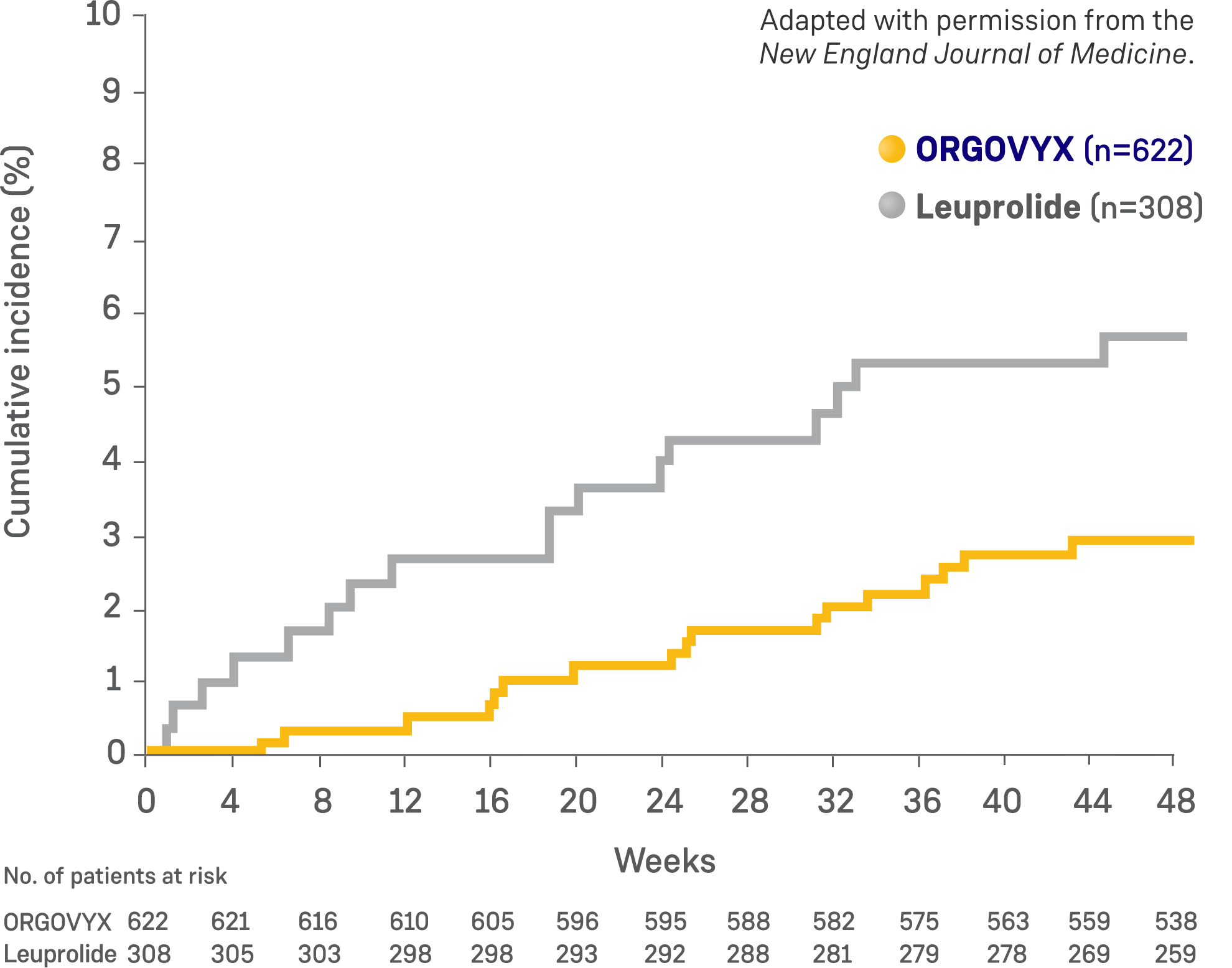
The incidence of major adverse cardiovascular events was a prespecified safety analysis. This was not a prospective efficacy endpoint in the study, the events were not adjudicated, and only descriptive analyses were performed. For these reasons, the FDA did not include the incidence of major adverse cardiovascular events for leuprolide in the label. The major adverse cardiovascular event data for ORGOVYX compared with leuprolide should be interpreted with caution and in this context. The study excluded patients with myocardial infarction or thromboembolic events within 6 months, arrhythmias, and uncontrolled hypertension.2
MOST COMMON ADVERSE EVENTS
ADVERSE REACTIONS (≥10%) OF PATIENTS WITH ADVANCED PROSTATE CANCER
WHO RECEIVED ORGOVYX IN HERO1
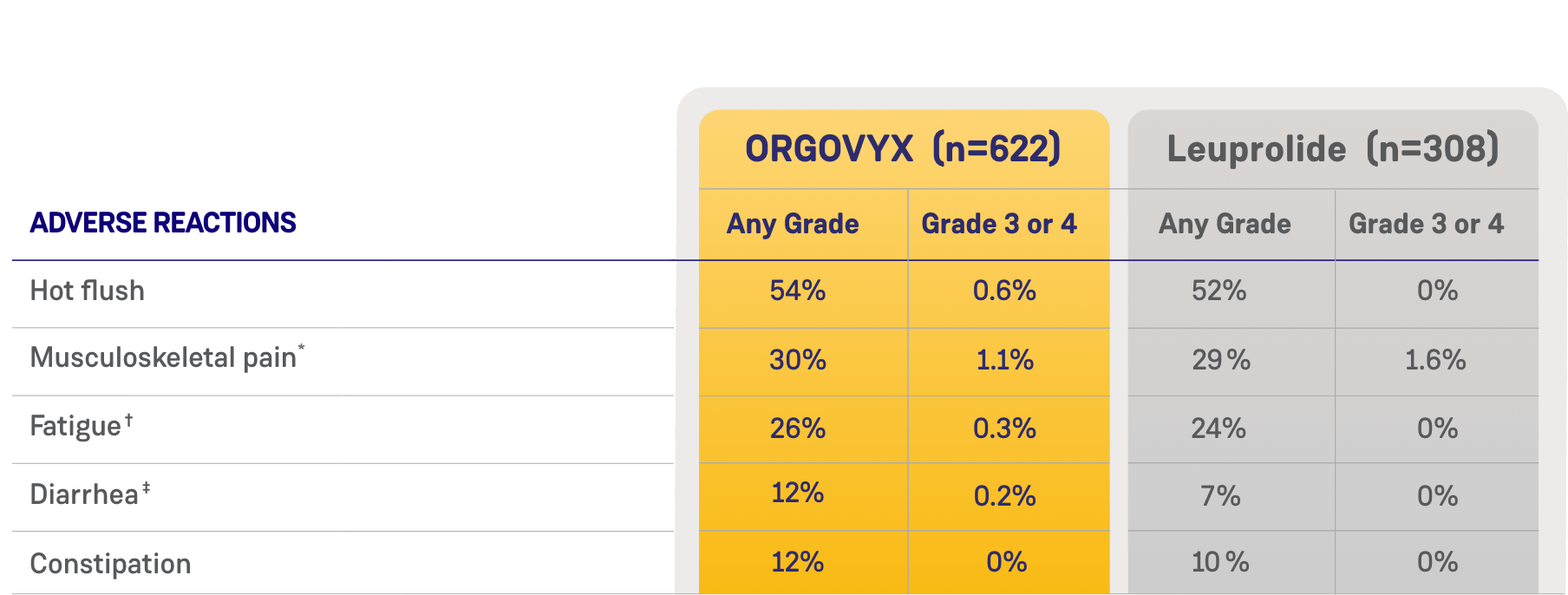
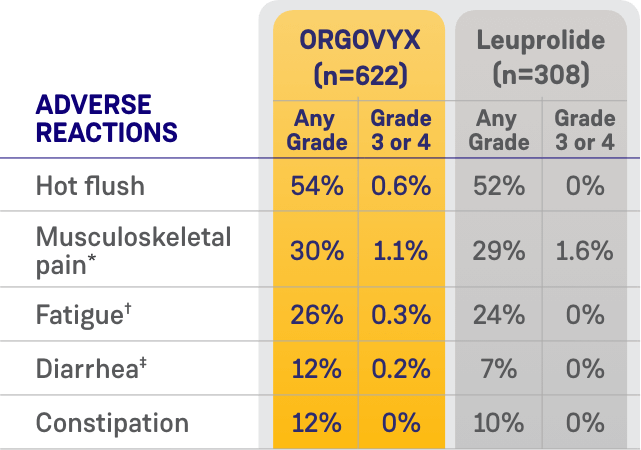
*Includes arthralgia, back pain, pain in extremity, musculoskeletal pain, myalgia, bone pain, neck pain, arthritis, musculoskeletal stiffness, noncardiac chest pain, musculoskeletal chest pain, spinal pain, and musculoskeletal discomfort.
†Includes fatigue and asthenia.
‡Includes diarrhea and colitis.
- Most common laboratory abnormalities (≥15%, all grades) in patients receiving ORGOVYX vs leuprolide were glucose increased (44% vs 54%), triglycerides increased (35% vs 36%), hemoglobin decreased (28% vs 29%), alanine aminotransferase increased (27% vs 28%), and aspartate aminotransferase increased (18% vs 19%)1
- Permanent discontinuation of ORGOVYX due to an adverse reaction occurred in 3.5% of patients1

ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or to any of the product components.
QT/QTc Interval Prolongation: Androgen deprivation therapy, such as ORGOVYX may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, or frequent electrolyte abnormalities and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Hypersensitivity: Angioedema was reported in 0.2% of patients treated with ORGOVYX in HERO. Hypersensitivity reactions, including pharyngeal edema and other serious cases of angioedema, have been reported in post-marketing with ORGOVYX. Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue ORGOVYX and promptly seek medical care. Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated.
Embryo-Fetal Toxicity: The safety and efficacy of ORGOVYX have not been established in females. Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX.
Laboratory Testing: Therapy with ORGOVYX results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after ORGOVYX may be affected. The therapeutic effect of ORGOVYX should be monitored by measuring serum concentrations of prostate-specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
Serious adverse reactions occurred in 12% of patients receiving ORGOVYX. Serious adverse reactions in ≥0.5% of patients included myocardial infarction (0.8%), acute kidney injury (0.6%), arrhythmia (0.6%), hemorrhage (0.6%), and urinary tract infection (0.5%). Fatal adverse reactions occurred in 0.8% of patients receiving ORGOVYX including metastatic lung cancer (0.3%), myocardial infarction (0.3%), and acute kidney injury (0.2%). Fatal and non-fatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX.
Most common adverse reactions (≥10%) and laboratory abnormalities (≥15%) in patients receiving ORGOVYX were hot flush (54%), glucose increased (44%), triglycerides increased (35%), musculoskeletal pain (30%), hemoglobin decreased (28%), alanine aminotransferase increased (27%), fatigue (26%), aspartate aminotransferase increased (18%), constipation (12%), and diarrhea (12%).
Co-administration of ORGOVYX with a P-gp inhibitor increases the area under the curve (AUC) and maximum concentration (Cmax) of ORGOVYX, which may increase the risk of adverse reactions associated with ORGOVYX. Avoid co-administration of ORGOVYX with oral P-gp inhibitors. If co-administration is unavoidable, take ORGOVYX first, separate dosing by at least 6 hours, and monitor patients more frequently for adverse reactions. Treatment with ORGOVYX may be interrupted for up to 2 weeks for a short course of treatment with certain P-gp inhibitors. If treatment with ORGOVYX is interrupted for more than 7 days, resume administration of ORGOVYX with a 360 mg loading dose on the first day, followed by 120 mg once daily.
Co-administration of ORGOVYX with a combined P-gp and strong CYP3A inducer decreases the AUC and Cmax of ORGOVYX, which may reduce the effects of ORGOVYX. Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers. If co-administration is unavoidable, increase the ORGOVYX dose to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX dose of 120 mg once daily.
ORGOVYX® (relugolix) is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer.
Please see full Prescribing Information for ORGOVYX.
- ORGOVYX (relugolix) [prescribing information]. Marlborough, MA: Sumitomo Pharma America, Inc.; 2024.
- Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187-2196 and supplementary material, available online.
- Data on file. Sumitomo Pharma America, Inc.
