- For US Healthcare Professionals Only
- Important Safety
Information - Indication
- Prescribing
Information - Patient
Website
of men achieved and maintained
testosterone suppression to
<50 ng/dL from Day 29 through
Week 48 with ORGOVYX (n=622)1
of men treated with leuprolide
(n=308) from Day 29 through
Week 481
THE HERO STUDY was a multinational, randomized, open-label, phase 3 trial in 930 men with advanced prostate cancer. Key inclusion criteria included men with advanced prostate cancer defined as biochemical prostate-specific antigen (PSA) or clinical relapse following local primary intervention with curative intent, newly diagnosed castration-sensitive metastatic disease, or advanced localized disease unlikely to be cured by local primary intervention, requiring at least 1 year of androgen deprivation therapy (ADT), ECOG 0/1. Patients were excluded if they had received previous systemic cytotoxic treatment for prostate cancer, a previous GnRH analog or other form of ADT >18 months total duration, or experienced significant cardiac conditions within 6 months before study entry.1-3
Patients were randomized 2:1 to receive ORGOVYX (360 mg on the first day followed by daily doses of 120 mg orally [n=622]) or leuprolide acetate (22.5 mg injection [or 11.25 mg† in Japan and Taiwan per local guidelines]) subcutaneously every 3 months (n=308) for 48 weeks.1,2
*The castration rate of the subgroup of patients receiving 22.5 mg leuprolide (n=264) was 88.0% (95% CI: 83.4%-91.4%).1†11.25 mg is a dosage regimen that is not recommended for advanced prostate cancer (APC) in the United States.1Please see additional information about ORGOVYX throughout this website.
HERO STUDY DESIGN
The efficacy and safety of ORGOVYX were evaluated in a multinational, phase 3, randomized, open-label, parallel-group study1,2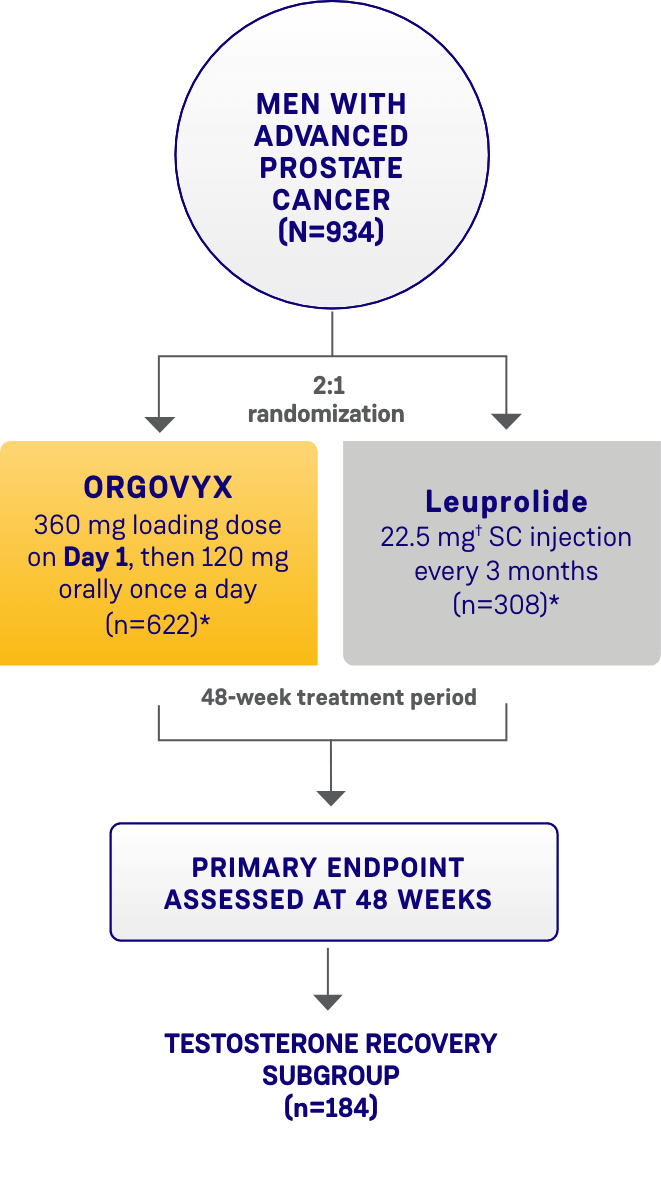
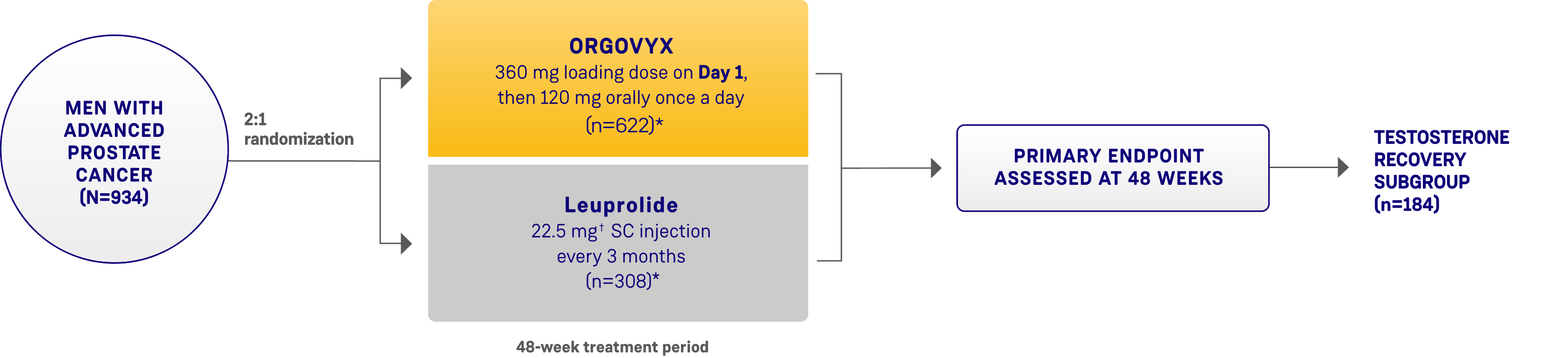
SC=subcutaneous.
*Two patients in each arm did not receive the study treatment and were not included.1
†The dosage of leuprolide was 11.25 mg in Japan and Taiwan, per local guidelines, and is not recommended for advanced prostate cancer in the United States.1
ENDPOINTS
Primary endpoint1:
- Sustained testosterone suppression rate (defined as the cumulative probability of testosterone suppression to <50 ng/dL while on study treatment from Day 29 through Week 48)
Key secondary endpoints include1,2:
- Testosterone suppression rates on Day 4 and Day 15 (defined as testosterone concentrations <50 ng/dL)
- PSA response rate on Day 15 (>50% decrease from baseline), confirmed on Day 29
- Profound testosterone suppression rate on Day 15 (defined as testosterone concentrations <20 ng/dL)
Testosterone recovery substudy1,2:
- Cumulative probability of testosterone recovery to 280 ng/dL at the 90-day follow-up in 184 patients who completed 48 weeks of treatment and who did not receive subsequent androgen deprivation therapy for at least 90 days after discontinuation
- This endpoint was analyzed for exploratory purposes without formal testing2
- 96.7% of men achieved and maintained testosterone suppression to <50 ng/dL from Day 29 through
Week 48 with ORGOVYX (n=622)- 88.8% of men treated with leuprolide (n=308) achieved and maintained testosterone suppression from Day 29 through Week 48
MAJOR EFFICACY OUTCOME MEASURE:
SUSTAINED TESTOSTERONE SUPPRESSION RATE
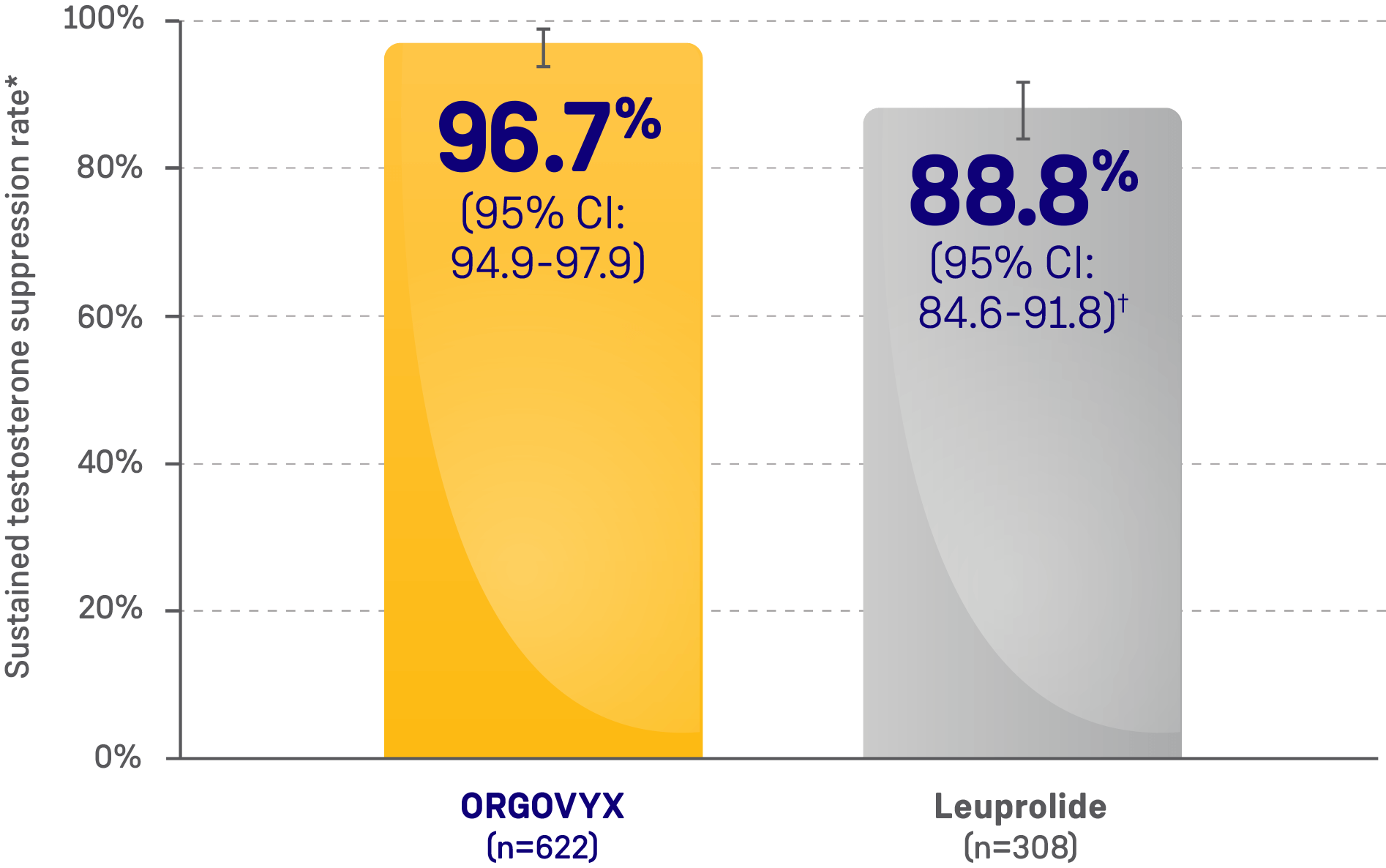
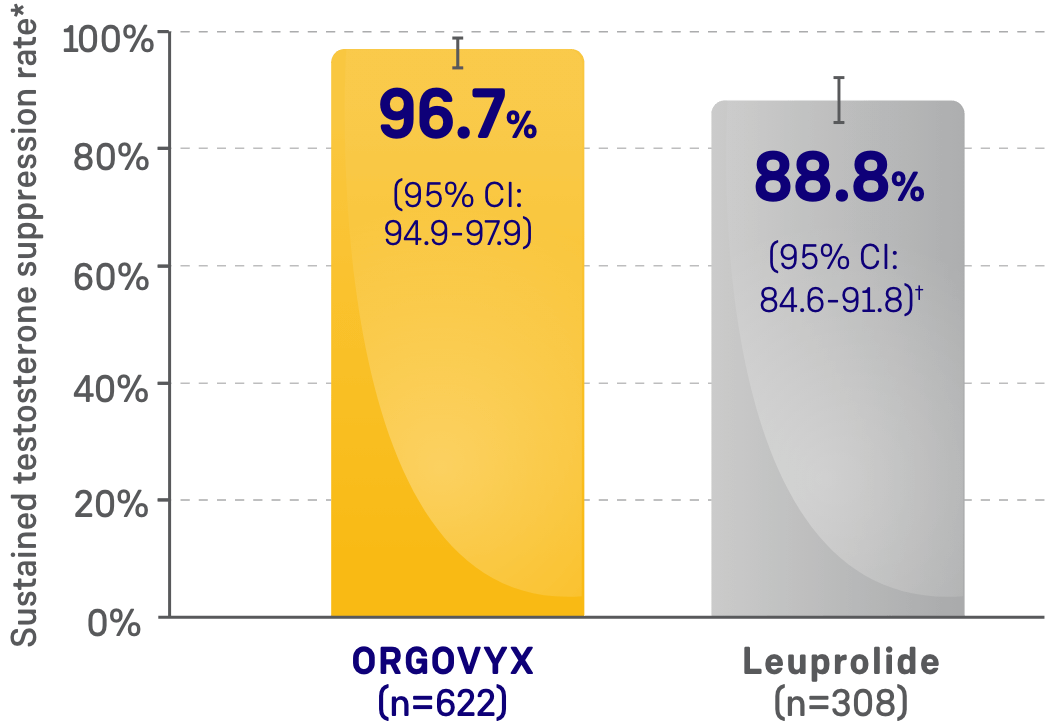
CI=confidence interval.
*Kaplan-Meier estimates within each group.
†The testosterone suppression rate of the subgroup of patients receiving leuprolide 22.5 mg (n=264) was 88.0% (95% CI: 83.4-91.4).
- On Day 4: 56% of men treated with ORGOVYX achieved testosterone suppression to <50 ng/dL*
- 0% of men treated with leuprolide had testosterone levels <50 ng/dL on Day 41
- On Day 15: 99% of men treated with ORGOVYX achieved testosterone suppression to <50 ng/dL*
- 12% of men treated with leuprolide had testosterone levels <50 ng/dL on Day 151
MEAN TESTOSTERONE CONCENTRATIONS FROM BASELINE THROUGH WEEK 482
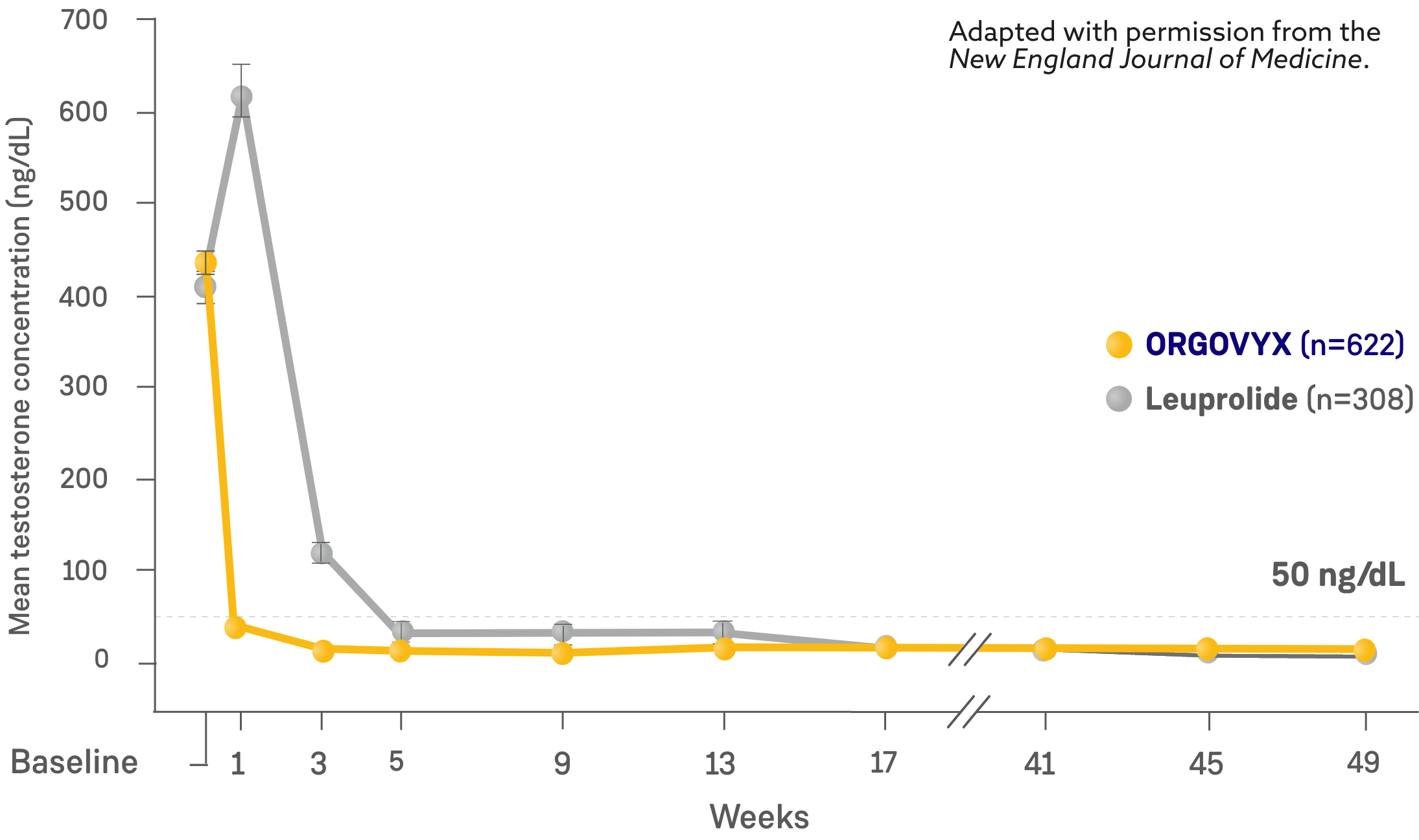
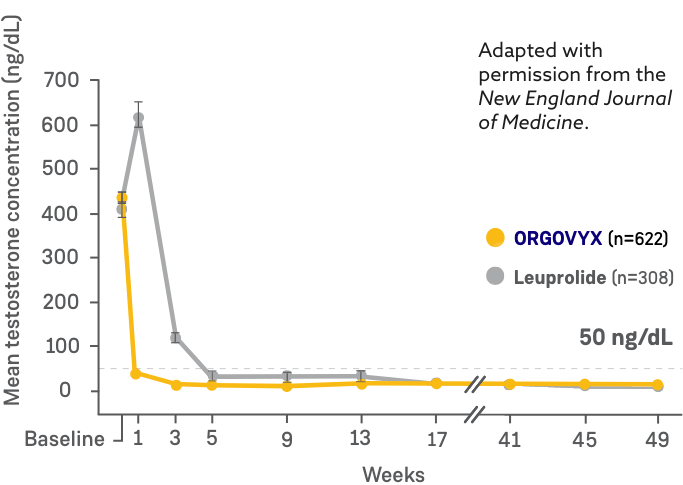
*Kaplan-Meier estimates within each group.
- On Day 15: 78% of men treated with ORGOVYX achieved profound testosterone suppression to <20 ng/dL*
- 1% of men treated with leuprolide had testosterone levels <20 ng/dL on Day 15
PERCENTAGE OF MEN WITH TESTOSTERONE
CONCENTRATIONS <20 ng/dL
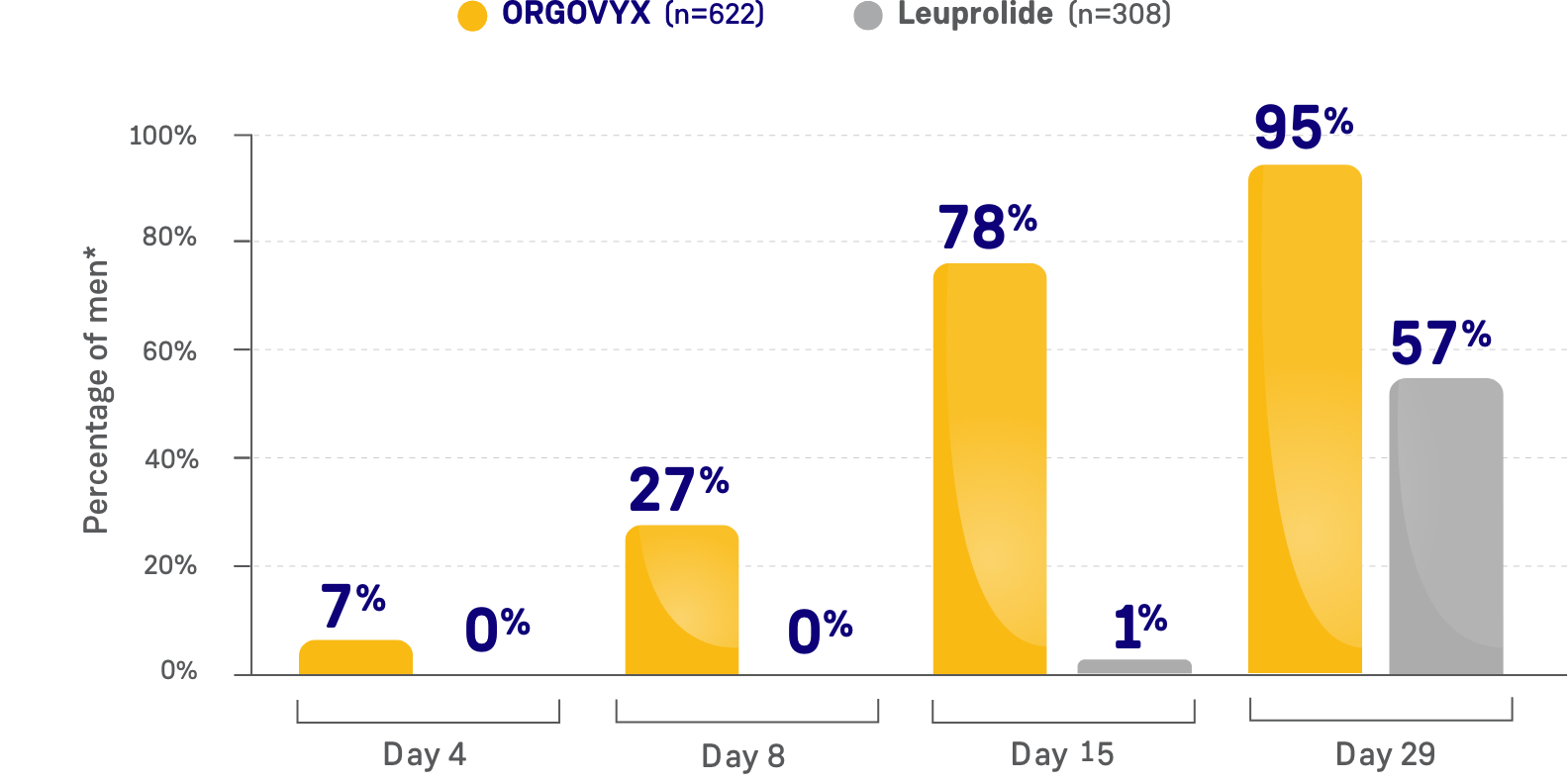

*Kaplan-Meier estimates within each group.
ORGOVYX DEMONSTRATED PSA REDUCTIONS FROM BASELINE
AVERAGE OF 104.2 ng/mL2
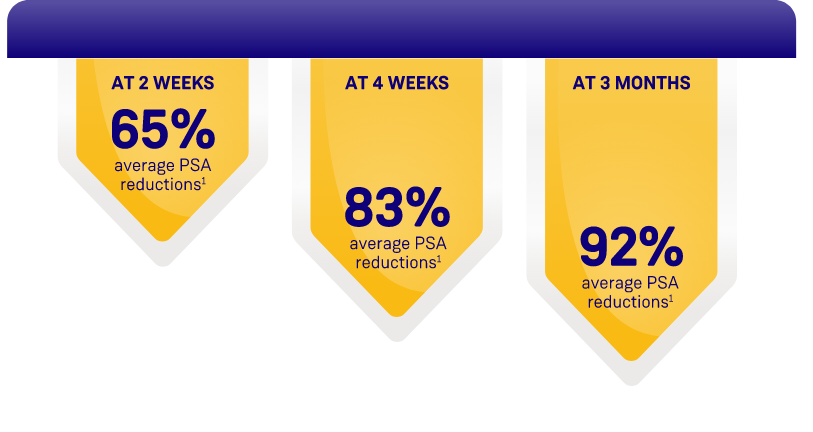
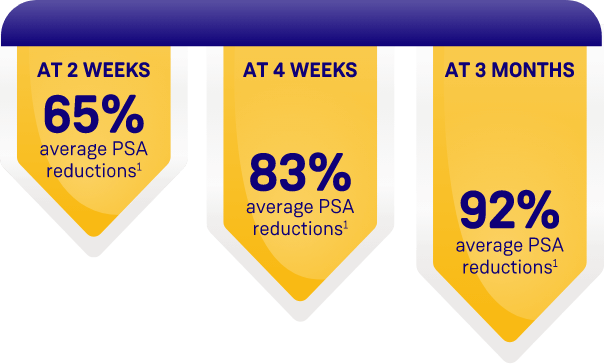
PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline is related to a clinical benefit.1
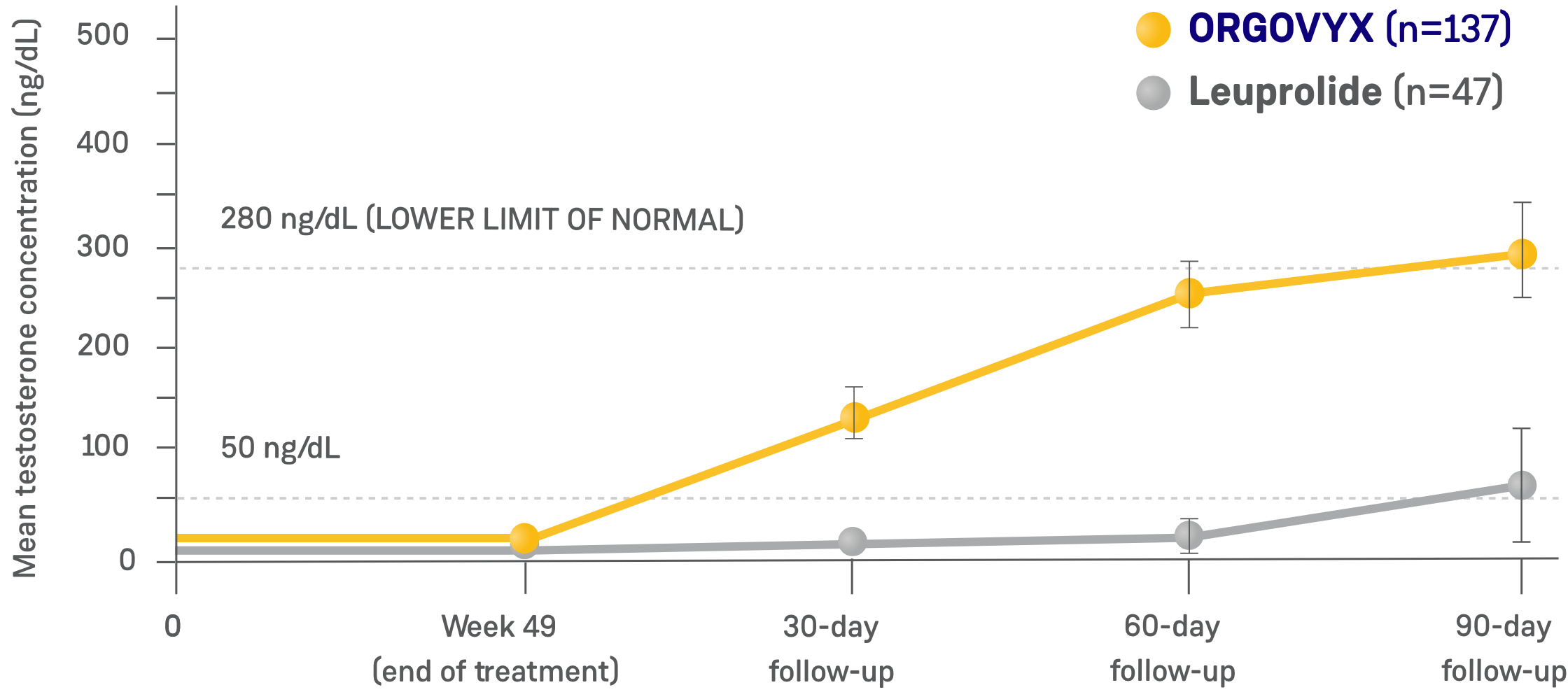
- Testosterone recovery was evaluated in a substudy of 184 patients who completed 48 weeks of treatment2*
- 90 days after treatment discontinuation, 55% of 137 men treated with ORGOVYX had their testosterone return to above the lower limit of the normal range (>280 ng/dL) or baseline values1,4*
- 3% of 47 men treated with leuprolide had their testosterone return to above the lower limit of the normal range (>280 ng/dL) or baseline values 90 days after discontinuation4
- This endpoint was analyzed for exploratory purposes without formal testing. The data from the leuprolide arm were not included in the US Prescribing Information for ORGOVYX1,2
*Kaplan-Meier estimates within each group.1
TESTOSTERONE CONCENTRATIONS IN TESTOSTERONE RECOVERY
SUBSTUDY (N=184)2
KEY INCLUSION CRITERIA1,2
- Men ≥18 years of age with confirmed adenocarcinoma of the prostate
- Requiring at least 1 year of continuous androgen deprivation therapy with 1 of the following clinical disease presentations:
- Evidence of biochemical (PSA) or clinical relapse following local primary intervention with curative intent
- Newly diagnosed androgen-sensitive metastatic disease
- Advanced localized disease unlikely to be cured by local primary intervention with curative intent
- Serum testosterone ≥150 ng/dL
- Serum PSA >2.0 ng/mL*
- ECOG score 0/1
KEY EXCLUSION CRITERIA2,3
- Patients likely to require chemotherapy or surgical therapy for symptomatic disease management within 2 months of initiating androgen deprivation therapy
- Previously received GnRH analog or other form of androgen deprivation therapy for >18 months total duration
- If androgen deprivation therapy was received for ≤18 months total duration, then patients must have completed treatment ≥3 months prior to baseline, or at least as long as the dosing interval of the depot formulation received
- Significant cardiovascular risk conditions
- Myocardial infarction or thromboembolic events within 6 months
- Arrhythmias
- Uncontrolled hypertension
ECOG=Eastern Cooperative Oncology Group; GnRH=gonadotropin-releasing hormone.
*When applicable, post radical prostatectomy of >0.2 ng/mL or post radiotherapy, cryotherapy, or high-frequency ultrasound >2.0 ng/mL above the postinterventional nadir.2
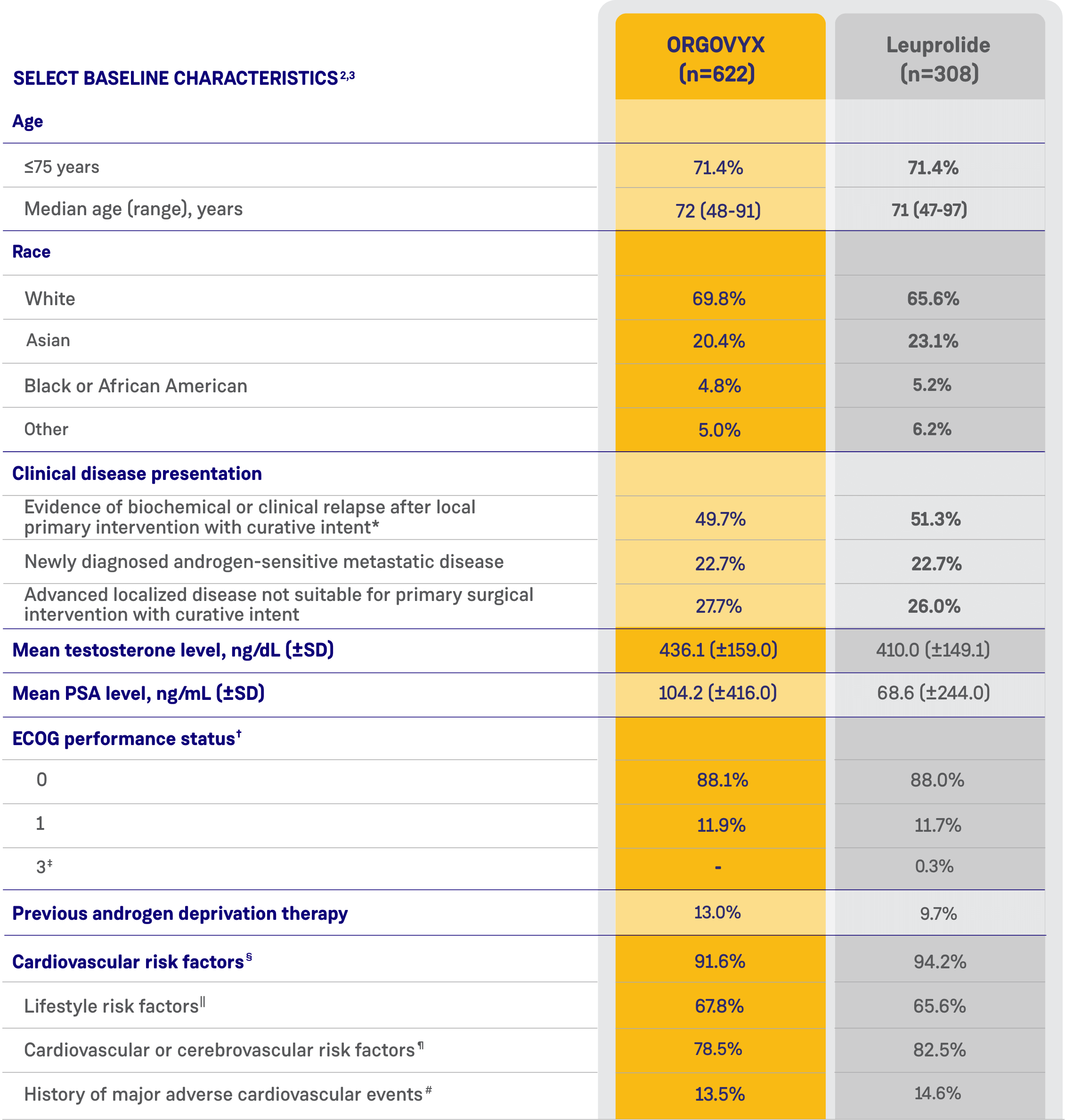
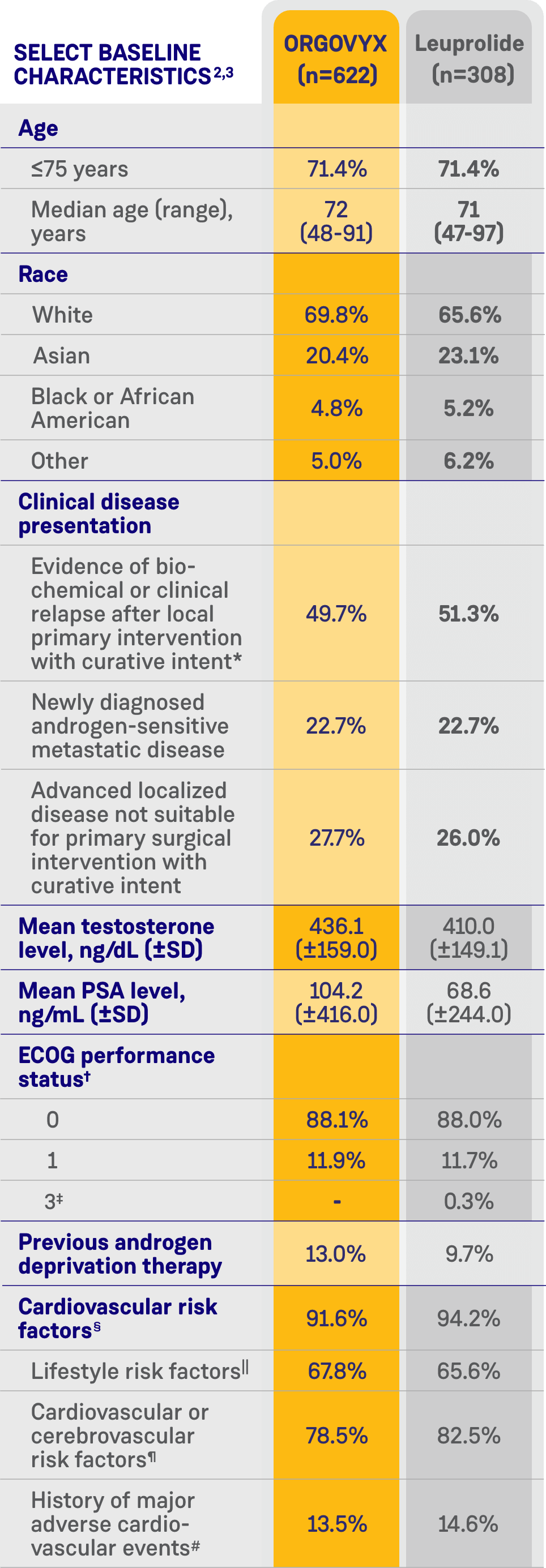
CARDIOVASCULAR RISK FACTORS AT BASELINE2
- 80% of men had cardiovascular or cerebrovascular risk factors such as diabetes and hypertension
- 67% had lifestyle risk factors such as a history of smoking or obesity
- 14% had a history of myocardial infarction or stroke
See Key Enrollment Criteria above for exclusion criteria about cardiovascular risk conditions.
*Biochemical relapse was defined by a rising PSA level.2
†ECOG performance status ranges from 0 to 5, with higher scores reflecting greater disability.2
‡One patient in the leuprolide group had a surgical vascular procedure on his leg and was given an ECOG score of 3 at screening because of the use of crutches. By the baseline Day 1 visit, the patient no longer used crutches, and his ECOG score had improved to 0.2
§Patients with multiple risk factors were counted only once.2
||Included current/past tobacco smoking, heavy alcohol use, and a BMI >30 kg/m2.2
¶Included hypertension; dyslipidemia; diabetes; a history of myocardial infarction or cardiovascular disease; a history of stroke, transient ischemic attack, or cerebral hemorrhage; peripheral arterial disease; atrial fibrillation and other arrhythmias; heart valve disease; chronic obstructive pulmonary disease; chronic kidney disease; chronic liver disease; carotid artery stenosis or occlusion; venous thromboembolic events; and heart failure.2
#Search criteria included "myocardial infarction" (broad standardized MedDRA query), "central nervous system hemorrhages and cerebrovascular conditions" (broad standardized MedDRA query).2
BMI=body mass index; MedDRA=Medical Dictionary for Regulatory Activities; SD=standard deviation.
IN YOUR AREA
*This coverage information is provided for informational purposes only; individual plans vary, and this may not include all plans. Sumitomo Pharma America and Pfizer make no representation or guarantee concerning coverage or reimbursement for ORGOVYX; please check with individual payers for plan-specific coverage and reimbursement information and requirements. Nothing herein may be construed as an endorsement, approval, recommendation, representation, or warranty of any kind by any plan or insurer referenced. This information is subject to change without notice. Data on file. Formulary data are provided by MMIT, LLC, as of December 2024. Transaction data are provided by SHS database as of December 2024.4

ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or to any of the product components.
QT/QTc Interval Prolongation: Androgen deprivation therapy, such as ORGOVYX may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, or frequent electrolyte abnormalities and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Hypersensitivity: Angioedema was reported in 0.2% of patients treated with ORGOVYX in HERO. Hypersensitivity reactions, including pharyngeal edema and other serious cases of angioedema, have been reported in post-marketing with ORGOVYX. Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue ORGOVYX and promptly seek medical care. Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated.
Embryo-Fetal Toxicity: The safety and efficacy of ORGOVYX have not been established in females. Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX.
Laboratory Testing: Therapy with ORGOVYX results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after ORGOVYX may be affected. The therapeutic effect of ORGOVYX should be monitored by measuring serum concentrations of prostate-specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
Serious adverse reactions occurred in 12% of patients receiving ORGOVYX. Serious adverse reactions in ≥0.5% of patients included myocardial infarction (0.8%), acute kidney injury (0.6%), arrhythmia (0.6%), hemorrhage (0.6%), and urinary tract infection (0.5%). Fatal adverse reactions occurred in 0.8% of patients receiving ORGOVYX including metastatic lung cancer (0.3%), myocardial infarction (0.3%), and acute kidney injury (0.2%). Fatal and non-fatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX.
Most common adverse reactions (≥10%) and laboratory abnormalities (≥15%) in patients receiving ORGOVYX were hot flush (54%), glucose increased (44%), triglycerides increased (35%), musculoskeletal pain (30%), hemoglobin decreased (28%), alanine aminotransferase increased (27%), fatigue (26%), aspartate aminotransferase increased (18%), constipation (12%), and diarrhea (12%).
Co-administration of ORGOVYX with a P-gp inhibitor increases the area under the curve (AUC) and maximum concentration (Cmax) of ORGOVYX, which may increase the risk of adverse reactions associated with ORGOVYX. Avoid co-administration of ORGOVYX with oral P-gp inhibitors. If co-administration is unavoidable, take ORGOVYX first, separate dosing by at least 6 hours, and monitor patients more frequently for adverse reactions. Treatment with ORGOVYX may be interrupted for up to 2 weeks for a short course of treatment with certain P-gp inhibitors. If treatment with ORGOVYX is interrupted for more than 7 days, resume administration of ORGOVYX with a 360 mg loading dose on the first day, followed by 120 mg once daily.
Co-administration of ORGOVYX with a combined P-gp and strong CYP3A inducer decreases the AUC and Cmax of ORGOVYX, which may reduce the effects of ORGOVYX. Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers. If co-administration is unavoidable, increase the ORGOVYX dose to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX dose of 120 mg once daily.
ORGOVYX® (relugolix) is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer.
Please see full Prescribing Information for ORGOVYX.
- ORGOVYX (relugolix) [prescribing information]. Marlborough, MA: Sumitomo Pharma America, Inc.; 2024.
- Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187-2196 and supplementary material, available online.
- Shore ND, Saad F, Cookson MS, et al. HERO phase 3 trial: Relugolix, an oral GnRH receptor antagonist, versus leuprolide acetate for advanced prostate cancer. Presented at: American Society of Clinical Oncology Virtual Scientific Program; May 29-June 2, 2020; virtual. Abstract 5602.
- Data on file. Sumitomo Pharma America, Inc.
